

|
|











AWARDS
All contents copyright © 2007 Readout Publications
|
Measurement, Control and Automation Resources throughout the World
| Eoin Ó Riain's impression's of the Werum MES Day held in Cork, Ireland 31st May 2007. |
Web pages of companies/speakers!
Report in Processingtalk MESA-Manufacturing Enterprise Solutions Association International Our Acronyms Page! |
"Miss Farber, would you please tell me what this piece of paper is doing on my desk?" |
Werum a company originating in Germany, concentrates it business on a few key areas and has earned a considerable reputation over the years. This year (2007) it was awarded the Frost & Sullivan North American Manufacturing Execution Systems Pharmaceutical & Biotechnology Industries Company of the Year Award. The areas it has developed are:
Test Data Management Systems; And Customized IT Solutions. In this conference the main emphasis was to get an inside perspective on the benefits of MES projects recently completed in Ireland. Learn from industry experts about what it takes to significantly improve the compliance & performance of pharmaceutical manufacturing processes. Further expansions of their PAS-X MES installations at Genzyme and Nycomed Pharma had recently been completed. The MES functionality they implemented covers all essential manufacturing business processes as well as the integration of the MES with the existing automation and ERP layers.
Diarmuid Quinn of ESP discussed the path to successful introduction of MES. This covered planning and step-by-step progressing of the project and he concluded by listing key success factors such as correct product/vendor selection, clear schedule (goals and timelines), communiication, management commitment, project team resourcing and empowerment and early definition of post go-live support model.
The second "practical" presentation was on the gWAP (genzyme Waterford Automation Program) by Éamonn Ó Mathúna and Kevin Brady of this plant. Again this was very interesting because here were a plant which was already there and which wanted to achieve paperless manufacturing supply in five areas of the operation. The described the intial planning, the people involved and their areas of expertese and how the changes fitted into the pyramid described in the earlier presentation. They went through the entire project pointing out the milestones achieved and the results. And as in the previous presentation a list of lessons leaarned was included. This concluded with the statement "Ensuring the paper goes!" This can be difficult as operators are so wedded to paper records that they are loath to see them go! Finally the very excellent day ended with a look into the future and was summarised in a series of four statements:  1. To fully achieve operational excellence and lean manufacturing strategies in pharmaceutical production you need independent, but integrated MES. 1. To fully achieve operational excellence and lean manufacturing strategies in pharmaceutical production you need independent, but integrated MES.
2. MES is a global roll-out manufacturing excellence strategy and no longer only a system. 3. The FDA paradigm change to QbD and PAT needs improved process understanding and control, managing the flexible design and control space by powerful product specifications in the MES. 4. In ten years time every pharmaaceutical company will operate paperless with electronic batch recording/EBR systems.
|
The site is mantained by Eoin Ó Riain and any suggestions or thoughts are more than welcome.
Suggestions should be mailed to readout@iol.ie. Other Contact Details
The Signpost has been honoured to receive a number of awards.







 A very interesting day was held recently in Cork by Werum. The called it a Pharma MES day and it was entitled "The Paperless Plant Has Arrived." Even the organisers were surprised at the attendence. Over seventy five people attended in a veritable cross section of everybody who is anybody in the pharamceutical manufacturing business in Ireland.
A very interesting day was held recently in Cork by Werum. The called it a Pharma MES day and it was entitled "The Paperless Plant Has Arrived." Even the organisers were surprised at the attendence. Over seventy five people attended in a veritable cross section of everybody who is anybody in the pharamceutical manufacturing business in Ireland.
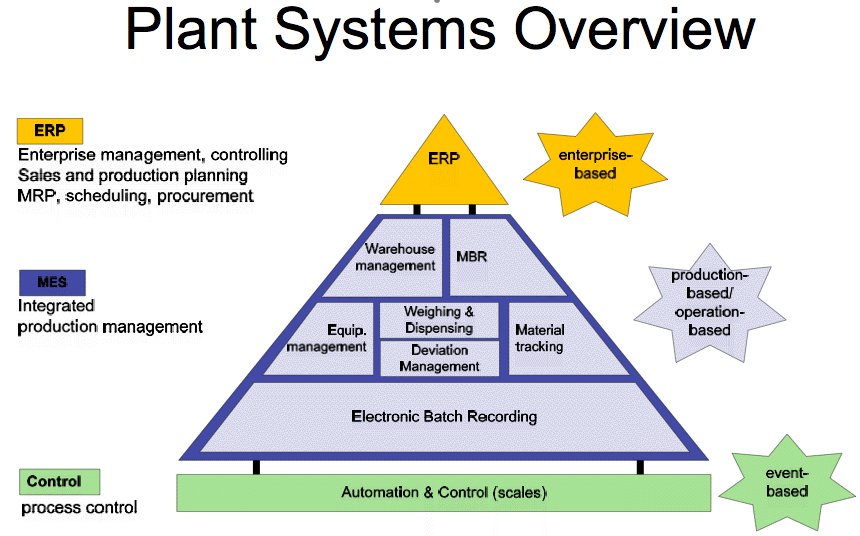 The conference opened with a brief overview of MES by Liam O'Brien of ESP - a partner company with Werum in Ireland. He dealt with the basics: What is MES? Factors influencing its selection and the benefits. He used a simple pyramid to show this. The base is the process automation and control in the plant which is broadly event based. On top of that the the integrated production management sector or MES is production or operation based. This contains functions such as batch recording, material tracking warehouse management etc. Finally there's the ERP area which is controlling the sales and production planning, scheduling and procurement.
The conference opened with a brief overview of MES by Liam O'Brien of ESP - a partner company with Werum in Ireland. He dealt with the basics: What is MES? Factors influencing its selection and the benefits. He used a simple pyramid to show this. The base is the process automation and control in the plant which is broadly event based. On top of that the the integrated production management sector or MES is production or operation based. This contains functions such as batch recording, material tracking warehouse management etc. Finally there's the ERP area which is controlling the sales and production planning, scheduling and procurement.

 Éamonn Ó Mathúna
Éamonn Ó Mathúna


